Insights of the maturity of quality management systems of the organizations for regulatory purposes
The European medical technology regulatory framework aims to be “robust, transparent, predictable and sustainable” ensuring safety. These objectives will be crystallized at the end of the transition period of regulations EU 2017/745 Medical Devices (MDR) and EU 2017/746 in vitro medical devices (IVDR). Manufacturers of medical devices technologies aim at placing their products into the European union must show evidence and ensure the devices are safe and effective under these European laws that came into force on May 26, 2022.
Both regulations prescribed the requirements that manufacturers and economic operators must fulfill. The key aspects to IVDR and MDR are the providing objective evidence that products are safe, effective, meet its intended use all trough product’s life cycle within a mature quality management system (QMS) and registration of economic operators and products in the European Database on Medical Devices (EUDAMED). The objective evidence is presented in the technical documentation (TD) including TD for post-market surveillance. MDR have 123 articles with XVII annexes and IVDR 113 articles with XV annexes describing a wide range of requirements.
Next, the manufacturer is responsible for the conformity assessment to IVDR/MDR for devices IVD class A and MD class I, whereas the Notify Body is involved in the conformity assessment for IVD classes B, C & D and for MD class I sterile (Is), I measurement (Im), IIa, IIb & III. The slow transition to the IVDR&MDR and the lack of availability of Notified Bodies are bottlenecks to have available IVD and MD compliant to IVDR and MDR. Hence, the European commission amended IVDR and MDR with regulations EU 2023/607 and EU 2024/1860 with conditions and timelines extended depending on the class until December 2028 (MD Is, Im, IIa, IIb) and September 2029 (IVD class B & A) to avoid shortage of devices and ensure an smooth transition.
Currently, exists over 161 difference “non-binding” guidance documents developed by Medical Device coordination group to present the “common understanding” of how the MDR and IVDR should be applied in practice. These MDCG guidances aim to help effective and harmonise implementation of IVDR&MDR. In addition, there are 29 different European harmonized standards that help manufacturers to determine the “presumption of conformity to IVDR and MDR”. The regulations and supporting documentation require manufacturers to enhance internal regulatory intelligence activities, have adaptability and resilience to cope with the regulatory constant updates.
In this study investigation, the manufacturers for IVD and MD showed their profiles, experiences, and maturity of QMS related to IVDR&MDR implementation. The quality method for research was a survey without incentives, nor funding and results analyzed using Webprol survey and data analysis package capabilities.
This study shows that manufacturers have a negative or neutral sentiment related to the governance and efficiency of IVDR/MDR. Similar results are observed by the study prepared by MedTech group (2024) where the recommendation is to improve predictability, timelines for Notified Body assessment.
This study shows two categories of challenges 1) challenges related to implementation of IVDR/MDR: high cost of implementation (up to 100 thousands of Euros) of IVDR/MDR, product reclassification, management of resources, issues with technical documentation and submissions, product discontinuation and update of agreements with economic operators 2) other challenges while implementing IVDR/MDR are related to pricing/profitability pressure, new product development, funding/credit/financing.
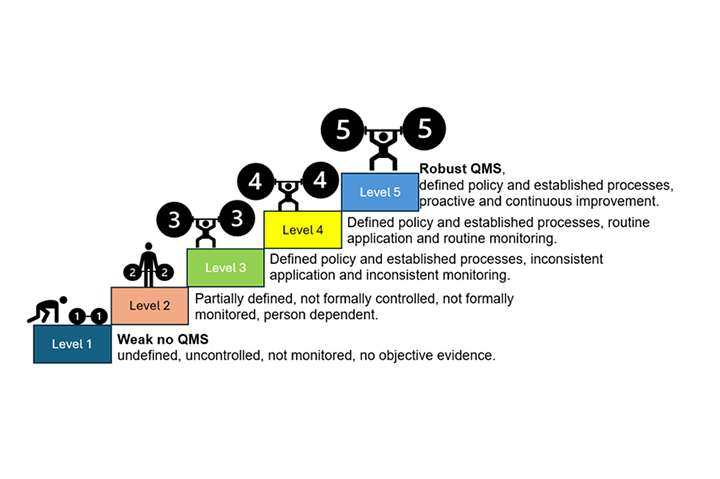
Another aspect study in this thesis is the assessment of maturity of QMS related to the responsibilities of manufacturers to IVDR/MDR Article 10. Maturity levels in quality is a methodology developed in 1979 (Crossby), exploited by different organizations with wide range of applications (ISPE, 2017) to set the stage for discussion towards improvement of QMS to bloom the maturity and to enhance management and stakeholder commitments. The five level of maturity Crosby& ISPE is presented in Figure 1.
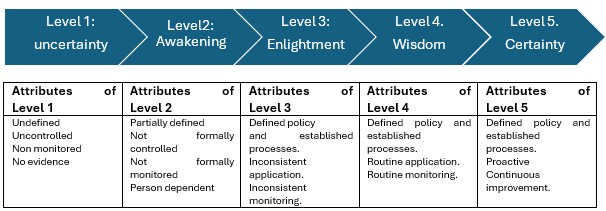
Figure 1: Quality Management System Maturity level based on Crosby (1979) and ISPE (2017)
The 12 participant organizations of the study have ISO13485 certificate and showed different maturity profiles for different QMS requirements required by IVDR&MDR. The profile of maturity ranges from level 3-5 and there is a low influence of company size in the profile.
The findings indicate that organizations are well-prepared to have a mature QMS expected by EU regulations. The critical factors to achieve level 4 and 5 beyond establishing and implementing a process is the Key Performance Indicators (KPI) use to monitor a process, based on the data of performance of the process, the organization can take informed decisions to improve the process.
In this study, the different organizations shared the KPI related to IVDR&MDR implementation being time, cost, risk and effort the preferred parameters to follow during IVDR&MDR implementation. The KPI chosen to monitor a process in a management system should be Specific, Measurable, Achievable, Relevant as suggested by Podgorski (2015),
This study has the following limitations: it lacks representatives of micro companies (less than 10 head count) also as the IVDR&MDR are under implementation, longitudinal studies should be taken into consideration to assess the maturity, and experiences during and at the end of the transition periods of IVDR&MDR.
References
Crosby, P.B. (1979) Quality Is Free: The Art of Making Quality Certain. McGraw-Hill, New York.
ISPE (2017) GAMP Guide: Records and Data Integrity. 147 pages
MedTech Europe (2024) Public Report December 2024: IVDR&MDR Survey Results 2024, pages 69
Podgorski, D. (2015). Measuring operational performance of OSH management system – A demonstration of AHP-based selection of leading key performance indicators. Safety science, 73, 146-166.
European Commission (2017) Regulation (EU)2017/745 of the European Parliament and of the Council of 5 April2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC)Nº178/2002 and Regulation (EC)Nº1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2017:117:FULL&from=EN
European Commission (2017) Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R0746&qid=1511869676775&from=EN